
Web`10^(-6)M HCl` is diluted to `100` times. Its `pH` is: WebThe solution dilution calculator tool calculates the volume of stock concentrate to add to achieve a specified volume and concentration. The calculator uses the formula M 1 V 1 =.
10 6 M Hcl Is Diluted To 100 Times, `10^(-6)M HCl` is diluted to `100` times. Its `pH` is:, 7.23 MB, 05:16, 1,397, Doubtnut, 2020-01-19T07:43:50.000000Z, 19, 10^-6 M HCl is diluted to 100 times its ph is - Chemistry - Chemical, www.meritnation.com, 1022 x 818, jpeg, ph hcl diluted times its chemical dear student, 20, 10-6-m-hcl-is-diluted-to-100-times, KAMPION
WebOn diluting $$100$$ times, volume becomes $$100L$$. Molarity= $$\cfrac {10^{-1}}{100}10^{-3}=[H^+]$$ $$\therefore pH= -\log [H^+]=3$$ WebHCl is lot of concentration or 32%,33%,36%etc,10 % means 10 ml in 100 ml 0f water. Molarity depends upon the weight. We able to HCl concentration as indicate above then. WebHint: $ {{10}^{-6}} $ M HCl is diluted to $ 100 $ times means water is added to it or we can say that due to addition of water the concentration of HCl decreases. So,. WebClick here 👆 to get an answer to your question ️ If a solution of 10^-6 m hcl is diluted 100 times the ph of solution is. veenat2013 veenat2013 01.12.2018 Chemistry. Web6. Like you guessed, the pH should be around 7, since the concentration of HCl is very low. Obviously, it can't be 11.65, since it's still an acid. The reason for this. WebOn dilution an acid solution never becomes neutral or basic. When on dilution H + becomes lesser than 10 - 7 , H + from water is also added. After dilution H + = 10 - 6 100 = 10 - 8. H. WebCommon ion effect is nothing but the Le Chatelier principle applied to ionic solutions).When the acid concentration is very low ( say, about 10^-6 M) the contribution of water to [H+]. Web\( 10^{-6} \mathrm{M} \mathrm{HCl} \) is diluted 100 times. Its \( \mathrm{pH} \) is :-(1) \( 6.0 \)(2) \( 8.0 \)(3) \( 6.95 \)(4) \( 9.5 \)\( P \)📲PW App L... Web10^-6 M HCL is diluted to 100 times .it's PH is 8 Advertisement Advertisement New questions in Chemistry. If it takes you 10 seconds to increase from 0/km/h to 50km/h.
Here `10^(-6)M HCl` is diluted to `100` times. Its `pH` is: more
Discussion , 10^-6MHCl is diluted 100 times. Its pH is :- (1) 6.0 (2) 8.0 (3) 6.95 (4) 9.5 P, , trending
Explanation of 10 6 M Hcl Is Diluted To 100 Times Next
`10^(-6)M HCl` is diluted to `100` times. Its `pH` is:
About 10^-6 M HCl is diluted to 100 times its ph is - Chemistry - Chemical Latest

Images 10^-6M HCl is diluted to 100 times. Its pH is: popular

New 10^(-6)M HCl is diluted to 100 times. Its pH is: trending

Currently - 0.1M HCl solution is diluted by 100 times The pH of the solution is: popular

Let's see Calculate pH of the (write the value to the nearest integer) Decimolar

Here One gram of CaCO3 was dissolved in dilute HCL and the solution dil viral

Discussion Solved What is the pH of an aqueous solution with a hydrogen | Chegg.com going viral
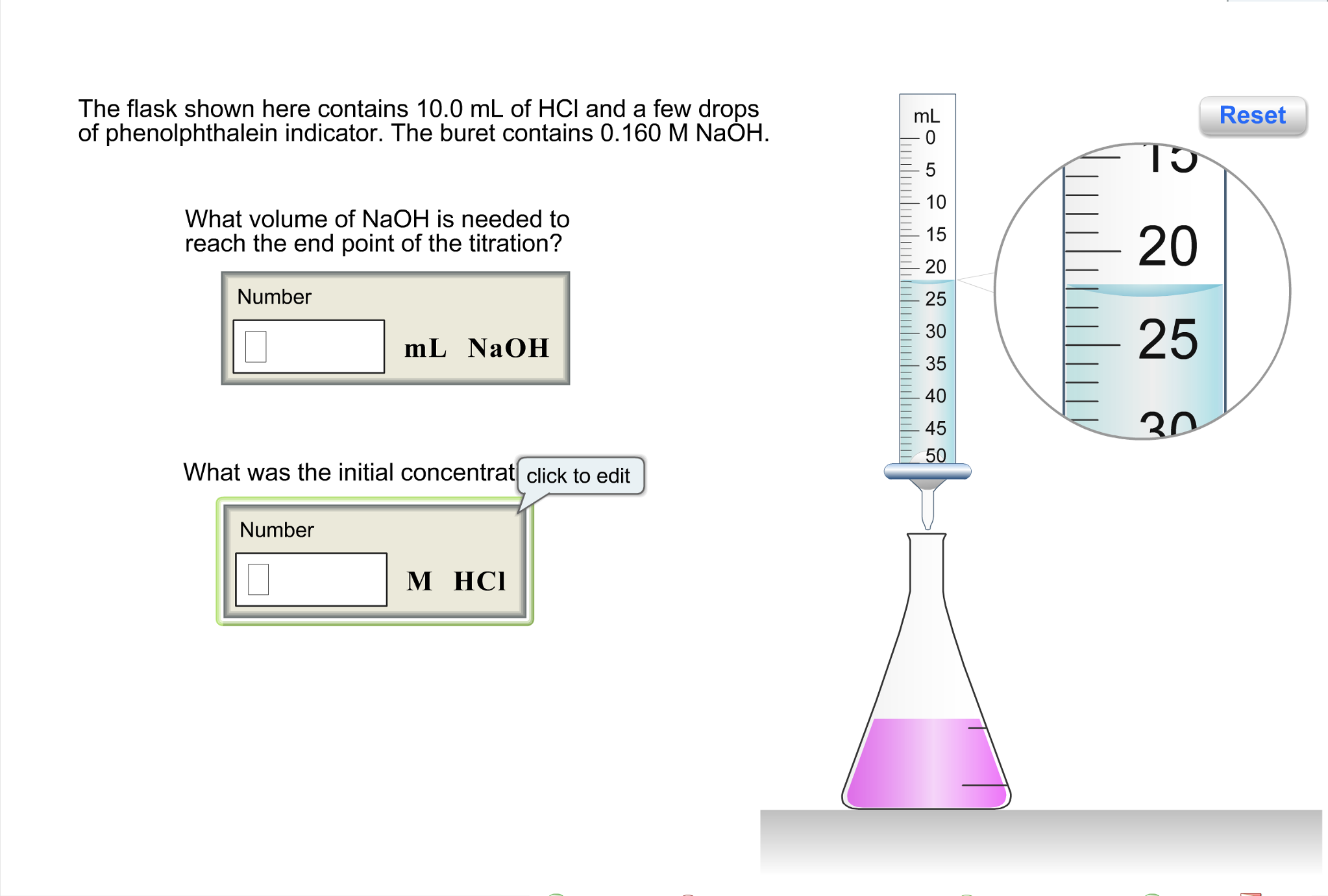
Photos 10^-6 M Na0H is diluted 100 times. The pH of the diluted base is popular

Viral HCl is labelled as 3.65 | Chemistry Questions viral

About 10^-6M HCl is diluted to 100 times. Its pH is:



Tidak ada komentar:
Posting Komentar