
Web>> A compound forms hexagonal close packed . Question . A compound forms hexagonal close packed structure. What is the number of octaheral voids in 0.5 mole of it? Medium.. WebThe reasons behind Hydrogen's hexagonal close-packed crystal structure aren't obvious, but that structure isn't very surprising either. Close-packing is the default behaviour when. WebOne can easily see that the closest packing of spheres in two dimensions is realised by a hexagonal structure: Each sphere is in contact with six neighboured spheres. Three.
A Compound Forms Hcp Structure, A compound forms hexagonal close-packed structure. What is the total number of voids in 0.5 mol of.., 6.59 MB, 04:48, 5,555, Dr. Bandhana Sharma, 2021-03-19T17:09:40.000000Z, 19, Estructura HCP - YouTube, www.youtube.com, 1280 x 720, jpeg, hcp estructura, 20, a-compound-forms-hcp-structure, KAMPION
WebA compound forms hexagonal close-packed structure Chapter 1: the Solid State Chemistry Class 12 solutions are developed for assisting understudies with working on their score. WebA compound forms hcp structure. Number of octahedral and tetrhedral voids in 0.5 mole 6f substance is respectively a. 3.011*1023, 6.022x1023 b. 6.022x1023,. WebThe Hexagonal Close-Packed (HCP) crystal structure is one of the most common ways for atoms to arrange themselves in metals. The HCP crystal structure is based on the. WebA compound forms hcp structure. Number of octahedral and tetrahedral voids in 0.5 mole of substance is respectively (a) 3.011 × 10 23, 6.022 × 10 23 (b). WebA compound forms hexagonal close packed structure. What is the total number of voids in 0.5 mol of it? How many of these are tetrahedral voids? Login. Study Materials . NCERT. WebIn hexagonal close packing (HCP) too, there are two basic kinds of voids are involved, namely, octahedral voids and tetrahedral voids. We know that the number of tetrahedral. WebNumber of close-packed particles = 0.5 × 6.022 × 10 23 = 3.011 × 10 23. Therefore, number of octahedral voids = 3.011 × 10 23. And, number of tetrahedral voids = 2 × 3.011 × 10 23. WebA compound forms hcp structure. The total number of voids in 0.5 mol will be- 1. 9.033×1023 2. 6.011×1023 3. 5.023×1023 4. 7.033×1023 The Solid State Chemistry. WebIn the HCP Structure, there are 6 corner atoms in the top layer and the bottom layer which makes it a total of 12, 2 atoms that base-centered, and 3 atoms that are there within the.
About A compound forms hexagonal close-packed structure. What is the total number of voids in 0.5 mol of.. more
Latest A compound is formed hexagonal close-packed structure. What is the total number of voids in 0.5 ... trending
A Compound Forms Hcp Structure that might be interesting
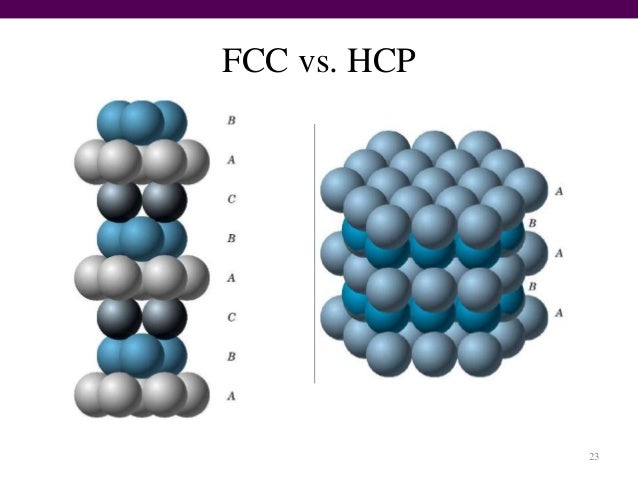
Intext Question 1.15 Page no. 23 THE SOLID STATE
A compound forms hexagonal close-packed structure. What is the total number of voids in 0.5 mol of it? How many of these are tetrahedral voids?
Estructura HCP - YouTube updated

Articles PPT - Types of Primary Chemical Bonds PowerPoint Presentation - ID:1586806 update
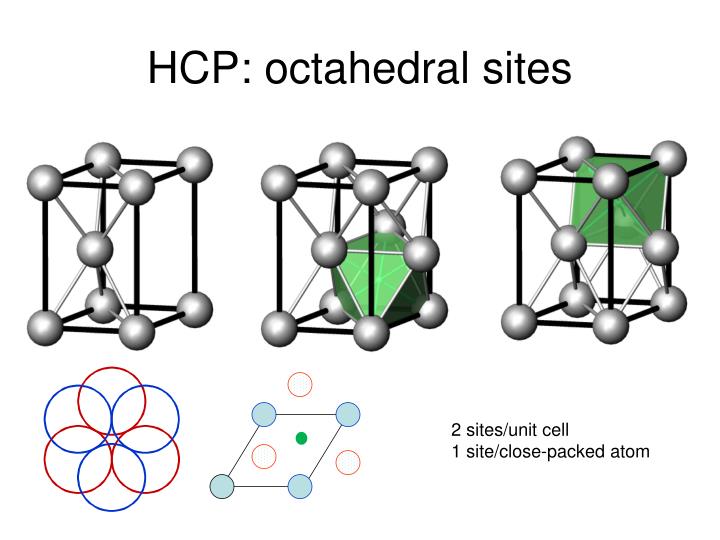
PPT - Types of Primary Chemical Bonds PowerPoint Presentation - ID:1586806
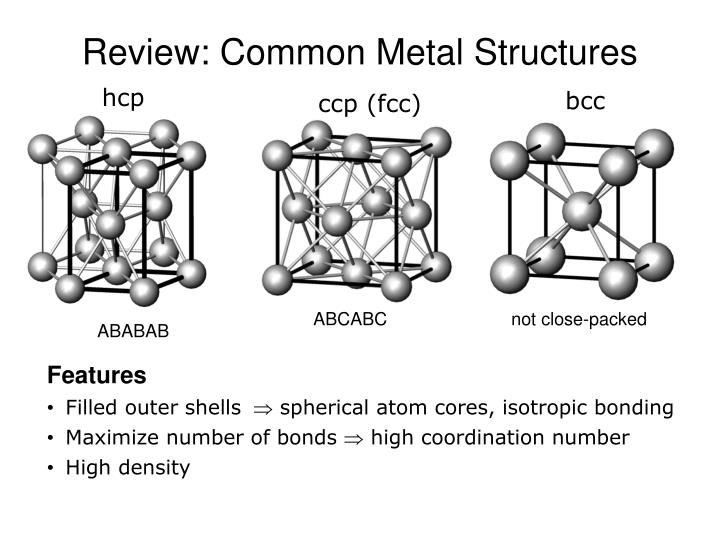
View How to code an array of FCC, BCC and HCP lattices in C++ - Stack Overflow trending

View hcp | Area | Crystal Structure viral

New In a common solid there will be many regions in the crystal, but there
New The Arrangement of Atoms in Crystalline Solids
Subject i need to find these two calculations for (HCP) | Chegg.com update
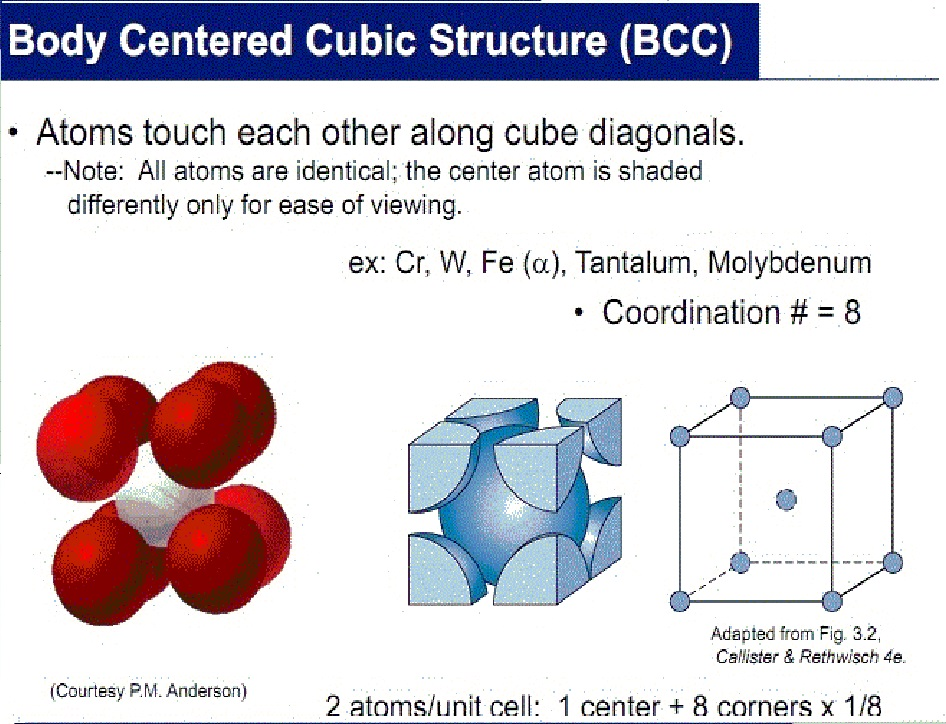
Let's see PPT - Crystal Structure Lecture 4 PowerPoint Presentation, free Latest
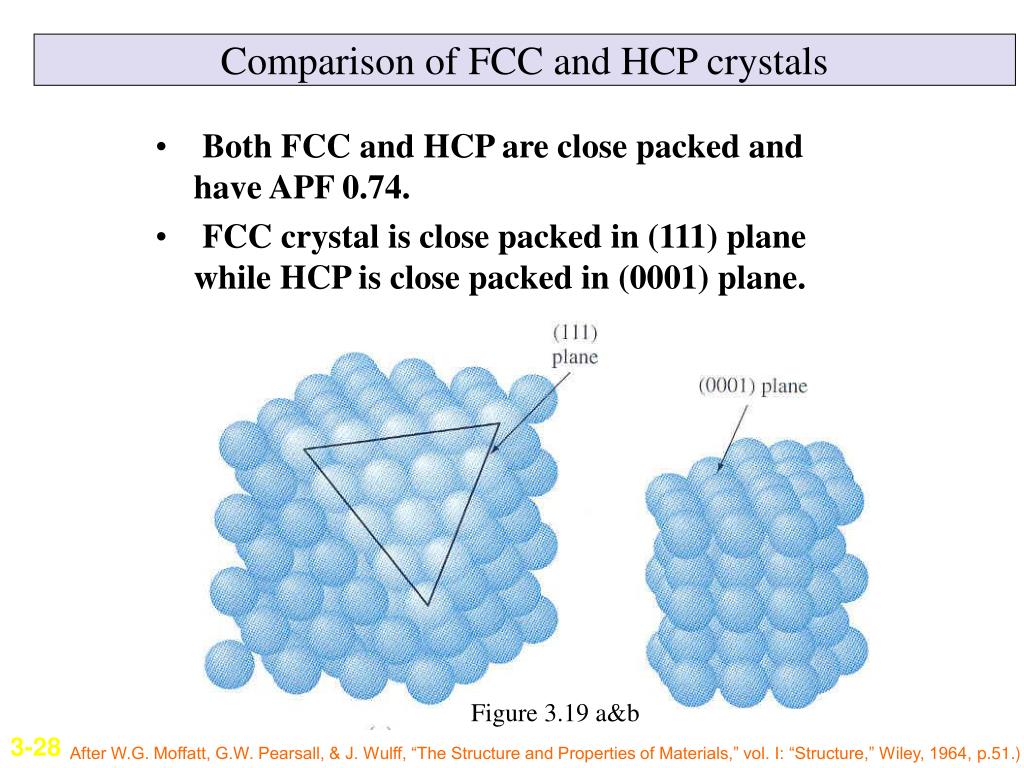
Module2 Latest


Tidak ada komentar:
Posting Komentar